0%
ছাড়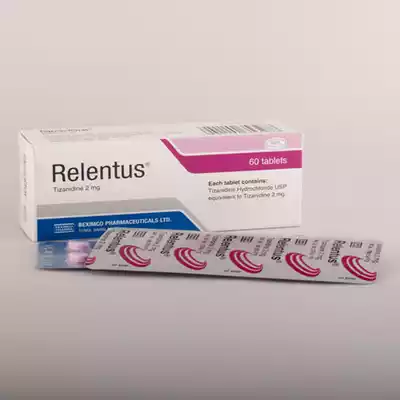

- Home
- /
- ফার্মেসি
- /
- ব্যথা ও জ্বর
বিস্তারিত
Indications
Treatment of painful muscle spasms:
Associated with static and functional disorders of the spine (cervical and lumbar syndromes).
Following surgery, e.g. for herniated intervertebral disc or osteoarthritis of the hip.
Treatment of spasticity due to neurological disorders:
Multiple sclerosis, chronic myelopathy, degenerative spinal cord diseases, cerebrovascular accidents, and cerebral palsy.
Pharmacology
Tizanidine is a centrally acting skeletal muscle relaxant. Its principal site of action is the spinal cord, where the evidence suggests that, by stimulating presynaptic alpha2 receptors, it inhibits the release of excitatory amino acids that stimulate N-methyl-D aspartate (NMDA) receptors.
Polysynaptic signal transmission at spinal interneuron level, which is responsible for excessive muscle tone, is thus inhibited and muscle tone reduced. In addition to its muscle-relaxant properties, tizanidine also exerts a moderate central analgesic effect.
Pharmacodynamic: Tizanidine is effective in both acute painful muscle spasms and chronic spasticity of spinal and cerebral origin. It reduces resistance to passive movements, alleviates spasms and clonus, and may improve voluntary strength. The antispastic activity (measured by the Ashworth score and pendulum test) and adverse effects (heart rate and blood pressure) of Tizanidine are related to plasma tizanidine concentrations.
Dosage & Administration
Tizanidine has a narrow therapeutic index and high inter-patient variability in tizanidine plasma concentrations which requires individualized dose adjustment. A low starting dose of 2 mg three times daily can minimize the risk for adverse effects. The dose should be carefully adjusted upward according to the needs of the individual patient.
Relief of painful muscle spasms: The usual dose is 2 to 4 mg three times daily in tablet form. In severe cases, an extra dose of 2 or 4 mg may be taken, preferably at night to minimize sedation.
Spasticity due to neurological disorders: The initial daily dose should not exceed 6 mg given in 3 divided doses. It may be increased stepwise at half-weekly or weekly intervals by 2 to 4 mg. The optimum therapeutic response is generally achieved with a daily dose of between 12 and 24 mg, administered in 3 or 4 equally spaced doses. The daily dose of 36 mg should not be exceeded.
Pediatrics patients: Experience in patients below 18 years of age is limited and the use of Tizanidine in this population is not recommended.
Geriatric patients (65 years of age or older): Experience with the use of Tizanidine in the elderly is limited. Therefore, it is recommended to start treatment at the lowest dose and increases should be done in small steps according to tolerability and efficacy.
Renal impairment: In patients with renal impairment (creatinine clearance <25 mL/min), it is recommended to start treatment at 2 mg once daily. An increase in dosage should be done in small steps according to tolerability and efficacy. If efficacy has to be improved, it is advisable to first increase the strength of the daily dose before increasing the frequency of administration.
Hepatic impairment: Use of Tizanidine in patients with severe hepatic impairment is contraindicated. While Tizanidine is extensively metabolized in the liver, limited data are available in this population. Its use has been associated with a reversible abnormality in liver function tests. Tizanidine should be used with caution in patients with moderate hepatic impairment and treatment should be started with the lowest dose. Afterward, an increase in dosage should be done carefully and according to patient tolerability.
Discontinuation of treatment: If Tizanidine has to be discontinued, the dosage should be slowly down titrated, particularly in patients who have received high doses for a longer period of time to avoid or minimize the risk of rebound hypertension and tachycardia.
* চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন'
Interaction
Concomitant administration of drugs known to inhibit the activity of CYP1A2 may increase the plasma levels of tizanidine. The increased plasma levels of tizanidine may result in overdose symptoms such as QT(c) prolongation. Concomitant administration of drugs known to induce the activity of CYP1A2 may decrease the plasma levels of tizanidine. The decreased plasma levels of tizanidine may reduce the therapeutic effect of Relentus.
Observed interactions resulting in a contraindication: Concomitant use of Relentus with fluvoxamine or ciprofloxacin, both CYP1A2 inhibitors is contraindicated. Concomitant use of Relentus with fluvoxamine or ciprofloxacin resulted in a 33-fold and 10-fold increase in tizanldine AUC, respectively. Clinically significant and prolonged hypotension may result along with somnolence, dizziness and decreased psychomotor performance. The increased plasma levels of tizanidine may result in overdose symptoms such as QT(c) prolongation.
Observed interactions resulting in concomitant use not recommended: Co administration of Relentus with other inhibitors of CYP1A2 such as antiarrhythmics (amiodarone, mexiletine, propafenone), cimetidine, fluoroquinolones (enoxacin, pefloxacin, norfloxacin), rofecoxib, oral contraceptives, and ticlopidine is not recommended.
Observed interactions to be considered: Caution should be exercised when Relentus is given with drugs known to prolong the QT interval (including but not limited to cisapride, amytriptyline and azithromycin).
Antihypertensives: Concomitant use of Relentus with antihypertensives, including diuretics, may occasionally cause hypotension and bradycardia. In some patients rebound hypertension and tachycardia have been observed upon abrupt discontinuation of Relentus when concomitantly used with antihypertensive drugs. In extreme cases, rebound hypertension might lead to cerebrovascular accident.
Rifampicin: Concomitant administration of Relentus with rifampicin results in 50% decrease in tizanidine concentrations. Therefore, the therapeutic effects of Relentus may be reduced during treatment with rifampicin, which may be of clinical significance in some patients. Long term coadministration should be avoided and if co- administration is considered a careful dose adjustment (increase) may be required.
Cigarette smoke: Administration of Relentus in smokers (>10 cigarettes per day) results in about 30% decrease in tizanidine systemic exposure. Long-term therapy with Relentus in heavy smokers may require higher doses than the average doses.
Alcohol: While on Relentus therapy, alcohol consumption should be minimized or avoided as it may increase the potential for adverse events (e.g. sedation and hypotension). The central nervous system depressant effects of alcohol may be enhanced by Relentus.
Anticipated interactions to be considered: Sedatives, hypnotics (e.g. benzodiazepine or baclofen), and another drug such as antihistamines may enhance the sedative action of tizanidine. Relentus should be avoided when using with other alpha-2 adrenergic agonists (such as clonidine) because of their potential additive hypotensive effect.
Contraindications
Known hypersensitivity to tizanidine or to any of the excipients.
Severely impaired hepatic function.
Concomitant use of tizanidine with strong inhibitors of CYP1A2 such as fluvoxamine or ciprofloxacin is contraindicated.
Side Effects
With low doses, such as those recommended for the relief of painful muscle spasms, somnolence, fatigue, dizziness, dry mouth, blood pressure decrease, nausea, gastrointestinal disorder and transaminase increase have been reported, usually as mild and transient adverse reactions.
With the higher doses recommended for the treatment of spasticity, the adverse reactions reported with low doses are more frequent and more pronounced, but seldom severe enough to require discontinuation of treatment. In addition, the following adverse reactions may occur: hypotension, bradycardia, muscular weakness, insomnia, sleep disorder, hallucination & hepatitis.
Psychiatric disorders: Common- Insomnia, sleep disorder.
Nervous system disorders: Very common- Somnolence, dizziness
Cardiac disorders: Uncommon- Bradycardia
Vascular disorders: Common- Hypotension
Gastrointestinal disorders: Very common- Gastrointestinal disorder, dry mouth; Common- Nausea.
Musculoskeletal and connective tissue disorders: Very common- Muscular weakness
General disorders and administration site conditions: Very common- Fatigue
Investigations: Common- Blood pressure decreased, transaminases increased.
Pregnancy & Lactation
As there is limited experience with the use of Tizanidine in pregnant women, it should not be used during pregnancy unless the benefit clearly outweighs the risk.
Animal data: Reproduction studies performed in rats and rabbits did not show evidence of teratogenicity. In rats, dose levels of 10 and 30 mg/kg/day increased gestation duration. Prenatal and postnatal pup loss was increased and development retardation occurred. At these doses, dams showed marked signs of muscle relaxation and sedation. Based on body surface area, these doses were 2.2 and 6.7 times the maximum recommended human dose of 0.72 mg/kg/day.
Lactation: Small amounts of tizanidine are excreted in rat milk. Since no human (fata are available Tizanidine should not be given to women who are breast-feeding.
Females and males of reproductive potential: Pregnancy testing: Sexually-active females of reproductive potential are recommended to have a pregnancy test prior to starting treatment with Tizanidine.
Contraception: Females of reproductive potential should be advised that animal studies have been performed showing Tizanidine to be harmful to the developing fetus. Sexually-active females of reproductive potential are recommended to use effective contraception (methods that result in less than 1 % pregnancy rates) when using Tizanidine during treatment and for 1 day after stopping treatment with Tizanidine.
Fertility: Animal data: No impairment of fertility was observed in male rats at a dose of 10 mg/kg/day, and in female rats at a dose of 3 mg/kg/day. Fertility was reduced in male rats receiving 30 mg/kg/day and in female rats receiving 10 mg/kg/day. Based on body surface area, these doses were 6.7 and 2.2 times the maximum recommended human dose of 0.72 mg/kg. At these doses, maternal behavioral effects and clinical signs were observed including marked sedation, weight loss and ataxia.
Precautions & Warnings
CYP inhibitors: The concomitant use of Relentus with moderate CYP1A2 inhibitors is not recommended. Caution should be exercised when Relentus is given with drugs known to increase the QT interval.
Hypotension: Hypotension may occur during treatment with Relentus and also as a result of drug interactions with CYP1A2 inhibitors and/or antihypertensive drugs. Severe manifestations of hypotension such as loss of consciousness and circulatory collapse have also been observed.
Withdrawal syndrome: Rebound hypertension and tachycardia have been observed after sudden withdrawal of Relentus, when it had been used chronically, and/or in high daily dosages, and/or concomitantly with antihypertensive drugs. In extreme cases, rebound hypertension might lead to cerebrovascular accident. Relentus should not be stopped abruptly, but rather gradually down titrated.
Hepatic dysfunction: Since hepatic dysfunction has been reported in association with tizanidine, but rarely at daily doses up to 12 mg, it is recommended that liver function tests should be monitored monthly for the first four months in patients receiving doses of 12 mg and higher and in patients who develop clinical symptoms suggestive of hepatic dysfunction, such as unexplained nausea, anorexia or tiredness. Treatment with Relentus should be discontinued if serum levels of SGPT or SGOT are persistently above three times the upper limit of the normal range.
Patients with renal impairment: In patients with renal impairment (creatinine clearance <25 mL/min) systemic exposure to tizanidine may increase up to 6 times compared to patient with normal renal function. Therefore, it is recommended to start treatment at 2 mg once daily.
Hypersensitivity reactions: Hypersensitivity reactions including anaphylaxis, angioedema, dermatitis, rash, urticarial, pruritis and erythema have been reported in association with tizanidine. Careful observation of the patient is recommended for one to two days after the first dose is administered. If anaphylaxis or angioedema with anaphylactic shock or difficulty of breathing is observed treatment with Relentus should be discontinued immediately and appropriate medical treatment should be instituted.
Driving and using machines: Patients experiencing somnolence, dizziness or any signs or symptoms of hypotension should refrain from activities requiring a high degree of alertness, e.g. driving a vehicle or operating machines.
Use in Special Populations
Renal impairment (creatinine clearance <25 mL/min): Maximal mean plasma levels were found to be twice as high as in normal volunteers, and the terminal half-life was prolonged to approximately 14 hours, resulting in much higher (approximately 6-fold on average) AUC values.
Hepatic impairment: No specific studies were conducted in this population. As tizanidine is extensively metabolized in the liver by the CYP1A2 enzyme, hepatic impairment may increase its systemic exposure. Relentus is contraindicated in patients with severe hepatic impairment.
Geriatrics (65 years of age and older): Pharmacokinetic data in this population are limited.
Gender: Gender has no clinically significant effect on the pharmacokinetics of tizanidine.
Ethnicity: The impact of ethnic sensitivity and race on the pharmacokinetics of tizanidine has not been studied.
Overdose Effects
In the few reports of Relentus overdosage received, recovery was uneventful, including by a patient who ingested 400 mg Relentus. Nausea, vomiting, hypotension, QT(c) prolongation, dizziness, somnolence, miosis, restlessness, respiratory distress, coma. It is recommended to eliminate the ingested drug by repeated administration of high doses of activated charcoal. Forced diuresis is expected to accelerate the elimination of Relentus. Further treatment should be symptomatic.
Therapeutic Class
Centrally acting Skeletal Muscle Relaxants
Storage Conditions
Store in a dry place below 30°C. Relentus must be kept out of the reach and sight of children.
Reviews (0)
Get specific details about this product from customers who own it.
This product has no reviews yet. Be the first one to write a review.
Your review
ভিডিও
Related Product
0%
ছাড়.webp)
 দেশাল-বাজার
দেশাল-বাজার
 চাল-ডালের দোকান
চাল-ডালের দোকান
 ইলেকট্রিক গ্যাজেট
ইলেকট্রিক গ্যাজেট
 কাচা বাজার
কাচা বাজার
 গ্রোসারি দোকান
গ্রোসারি দোকান
 ভোজনশালা
ভোজনশালা
 রান্নাঘর
রান্নাঘর
 পশুখাদ্য
পশুখাদ্য
 ফার্মেসি
ফার্মেসি
 মা ও শিশু'র পণ্য
মা ও শিশু'র পণ্য
.jpg)




.webp)


























.jpg)
.png)
.png)





.jpg)
.jpg)
.jpg)
-(1)-(1).png)
-(3)-(7)-(2).jpg)
-(13).jpg)
-(3)-(6).jpg)
-(12)-(2).jpg)
-(12)-(2)-(1).jpg)